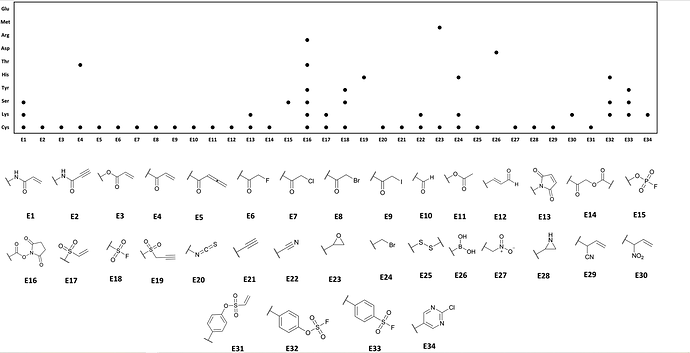
The response to our first call for designs was truly overwhelming - clearly this initiative hit a nerve, and a lot of careful thinking is clearly evident in the designs.
One thing though that was hugely underrepresented was designs of covalent compounds - and yet we see this as one of the major opportunities afforded by the experiment results.
Therefore, for the second call, we ask contributors to focus on designs that build on the covalent hits, especially ways of merging them with with the non-covalent hits, preferably multiple ones at the same time.
If you haven’t already, read Resnick et al 2019 for the full rationale and some examples of what we mean. The general premise is that, if whatever is attached to the covalent warhead is itself pretty potent, then the warhead can be very unreactive (and thus selective and non-toxic),et still form the covalent bond and thus inhibit with high efficiency.
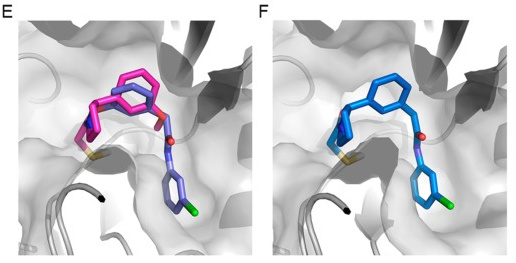
Figure 5E and F in Resnick et al 2019 capture what we hope to achieve; in that paper, a single merging step led to a dramatic improvement in potency.
Another variation is to replace the warhead with some other weakly reactive covalent group; feel free to suggest ideas acade we will be (computationally) exploring some replacements anyway, but feel free
(The covalent compounds are clearly marked in Excel file near the top of the download page of Diamond website. )
Thanks again for your enthusiasm…
Frank