5.48:yes!
https://www.drugbank.ca/drugs/DB08880
Give me a shout-what dilution conditions? And an address,; I can go in and get the compounds and send them. Hope all is well. Thx j
I’ve prepared some notes on design themes based on the 6y2f PDB structure that I hope will be helpful.
Thanks @pwkenny, this is an interesting read, and I will definitely send it along to a couple of others who were looking into the structures. One slight annoyance is that synthesis of these peptidomimetic molecules is relatively slow. Perhaps a naive question, but is there any efficient way to test these warhead relationships without synthesizing these entire large molecules?
And @JHullaert, wow are you fast already submitting some suggestions based on the paper https://covid.postera.ai/covid/submissions/86445305-1261-40d4-a2e8-d915c38a0708
You may be able to use a fragment-based approach to test warhead relationships if the warheads bind reversibly and you’re able to quantify weak affinity (this would also enable the structure-activity relationship for the P1 substituent to be explored). The assay is key and, ideally, it would be possible to directly measure affinity using a biophysical assay such as SPR. Nevertheless, biochemical assays can be run at high concentration if the appropriate precautions are taken and this article by former AZ colleagues may be helpful:
https://doi.org/10.1177%2F1087057109341768
This 1971 article gives some Ki values that were measured for some structurally-prototypical covalent inhibitors of papain:
@RGlen wrote up some nice nights on the recent feline coronavirus inhibitor paper that looks promising. Thought I would share with you all.
“”"
Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication.
- Inhibitors of SARS-COV2 that work in SARS_COV-2
- Compounds are in use in cats to cure FCoV.
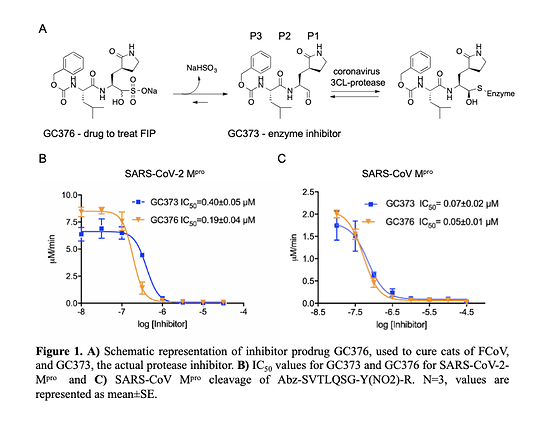
- GC376, and its analog GC373, are effective inhibitors of the Mpro from both SARS-CoV and SARS-CoV-2 with IC50 values in the nanomolar range.
- Crystal structures of the SARS-CoV and SARS-CoV-2 Mpro with these inhibitors have a covalent modification of the nucleophilic Cys145. NMR analysis reveals that inhibition proceeds via reversible formation of a hemithioacetal
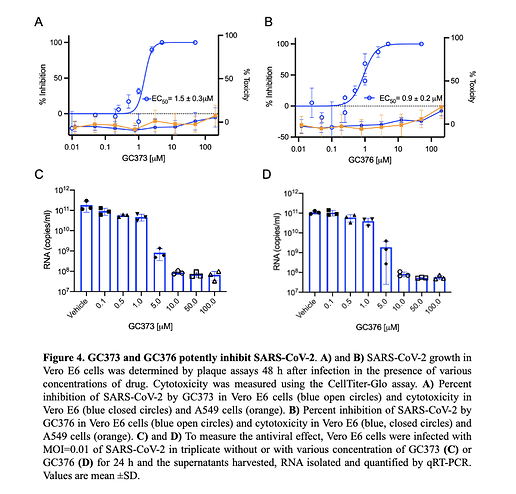
- Compounds show efficacy in Vero cells (effective about 5uM) with low toxicity and a therapeutic ratio of about 200.
- Protease inhibitors are common drug candidates if they
- meet the requirements of low toxicity, solubility, and reversibility.
- Several proteases have been identified as molecular targets and used for the development of novel classes of drugs including Tipranavir for the treatment of HIV.
- However, inhibition of cysteine proteases by thiol reactive species is often untenable for human drugs unless the inhibitor is reversible. Michael acceptor drugs that are irreversible in vivo, such as Rupintrivir, have failed in clinical trials due to low bioavailabilty. Undesired irreversible reaction occurs with numerous mammalian thiols to destroy the inhibitor.
- Reaction with host protein thiols could also potentially lead to acute toxicity or immune reaction.
- The pro-drug is a bit more active
It’s a carbamate, which are sometimes metabolically labile. Human carboxylesterases 1 and 2 in the liver cleave the ester bond of the carbamate. However some recent designs are stable to protease action and can be used as amide replacements. Metabolic stability of carbamates decreased (i.e., their metabolic stability increased), in the following series: Aryl-OCO-NHAlkyl >> Alkyl-OCO-NHAlkyl ∼ Alkyl-OCO-N(Alkyl)2 ≥ Alkyl-OCO-N(endocyclic) ≥ Aryl-OCO-N(Alkyl)2 ∼ Aryl-OCO-N(endocyclic) ≥ Alkyl-OCO-NHAryl ∼ Alkyl-OCO-NHAcyl >> Alkyl-OCO-NH2 > Cyclic carbamates.
Structure 6WTJ is on hold prior to release at PDB.
“”"