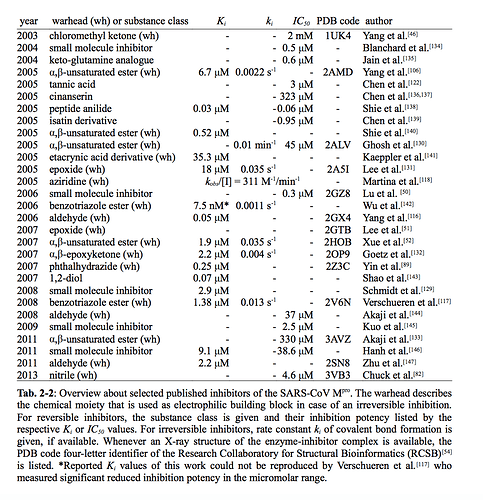
Wow @jarvist, thanks! This table is super useful.
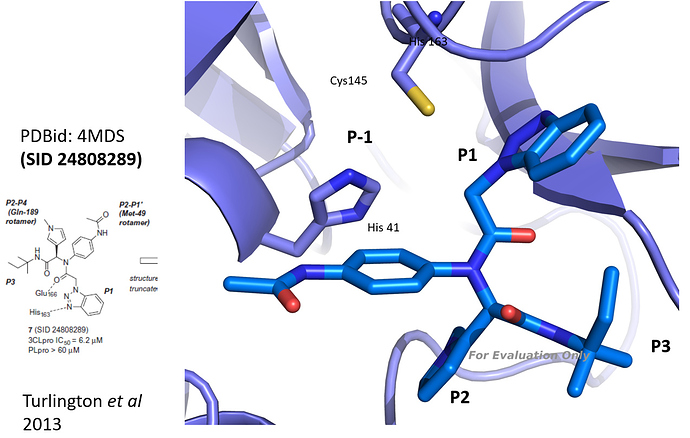
@mc-robinson I took the 3D structure of SID 24808289 (Turlington et al 2013, PDBid: 4mds, see figure) and compared it to the results of the analog search you performed. As you already pointed out, the series is quite interesting since representatives can bind with high potency even though not covalent. Based on a visual check (and a bit of imagination), I made a selection (in the attached powerpoint). I would keep the benzotriazole (which binds to Cys 145), and the amide and tertiary amine and then bring in diversity in the hope to find a molecule that addresses the P-1, P2 or P3 pockets efficiently. Hope this helps. Let me know - best Joost
existing_inhibitors_Enamine_selection_JU.pdf (455.3 KB)
Thank you @JoostU, this is indeed incredibly helpful and many of these should be ordered pretty soon.
Benzotriazole has a pKa of 8, is known to be activated via general acid catalysis and well positioned to react with CYS145. Can you really exclude formation of a covalent linkage with CYS145? Did the authors do experiments to exclude this hypothesis (apart from this XRD structure)?
Hi all,
In trying to answer the question above, I came across two reviews by Pillaiyar et al., who weren’t mentioned yet as far as I know
Great and extensive review on SARS protease targeting:
https://pubs.acs.org/doi/pdf/10.1021/acs.jmedchem.5b01461
And a very recent, more broad overview by the same author
In the last work I encountered AG-7088/ rupintrivir, a Pfizer drug that was already tested against the SARS protease in 2003. It made it as far as Phase II.
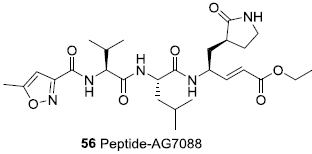
It has a lactam in P1 and a warhead (see above), so might have good activity. It can be purchased here
https://www.axonmedchem.com/product/1571
@JHullaert. Nice remark. I reread Turlington et al. and they did not do further experiments to look at reactivity with Cys 145. Also found the work below, presenting new benzotriazole series in the wake of the Turlington work. But IC50s are disappointing there (> micromolar). Actually only Turlington’s compound 17a (50 nM) has any decent potency. If it was a general reaction between benzotriazoles and Cys 145, I would expect a bit better IC50s overall. Nevertheless, 17a remains interesting, I think. An X-ray structure would help.
![]()
(Of course the benzotriazole series we talk about have a different binding mode than the suicide inhibitors with benzotriazole as a leaving group that are also mentioned above, or could 17a have a suicide inhibitor-like mechanism?)
Thanks @JoostU, 17A is currently being made, so hope to get it in a crystal structure soon. Great set of notes too, so thank you . The warhead of rupintrivir is quite similar to what is seen in https://patents.google.com/patent/US20050143320A1/en
and
https://patents.google.com/patent/US20060014821A1/en
(abandoned Pfizer patent)
But I need to do some more reading. Note that the first one quotes 75 of the compounds have sub-micromolar affinities.
Somebody may have already flagged these up but, just in case not, there are a couple of aldehydes (IC50 values of 40 nM and 50 nM) and there are crystal structures although these don’t seem to have been deposited yet: https://doi.org/10.1101/2020.03.25.996348
This the pdb:6LU7 crystal structure may be helpful.
The structure shown with the 30 nM Ki could be functioning as acylating agent (electron-poor aromatic ring facilitating nucleophilic attack on anilide cabronyl) and it’d be good to get crystal structure so that we can get a better idea of binding model. The chlorine looks like a good halogen bond donor. If this is the case then [Cl->Br] would be expected to lead to an increase in affinity. Oxidation of the catalytic cysteine thiol is always a concern when studying non-covalent binding to cysteine proteases).
Thanks @pwkenny, I see they just released a new crystal structure on PDB today of the same complex, 7BQY
Just posting images of the compounds so people don’t have to dig in SI.
11a 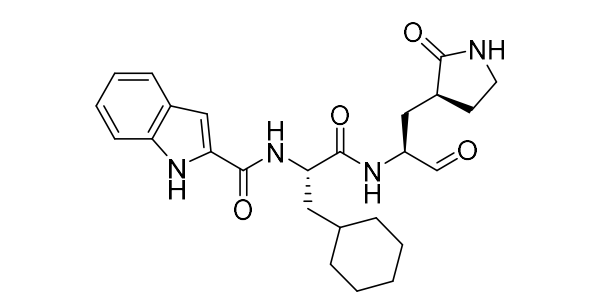
11b 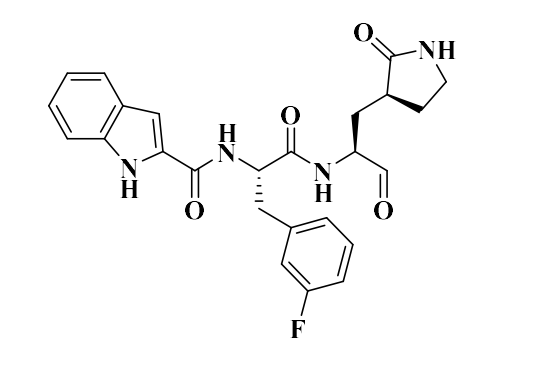
Which will eventually be released as PDBs 6LZE and 6M0K eventually.
Note that this biorxiv article @pwkenny flagged was published in Science today https://science.sciencemag.org/content/sci/early/2020/04/21/science.abb4489.full.pdf
Novel warheads with good ADME Tox profile just published. Maybe one to consider when refining warheads:
“Unfortunately, all existing inhibitors of GPX4 act covalently via a reactive alkyl chloride moiety that confers poor selectivity and pharmacokinetic properties. Here, we report our discovery that masked nitrile-oxide electrophiles, which have not been explored previously as covalent cellular probes, undergo remarkable chemical transformations in cells and provide an effective strategy for selective targeting of GPX4. The new GPX4-inhibiting compounds we describe exhibit unexpected proteome-wide selectivity and, in some instances, vastly improved physiochemical and pharmacokinetic properties compared to existing chloroacetamide-based GPX4 inhibitors”
Act on Se-Cys in paper but why not Cys? I also seem to recall a number of “propellor” antihistame like similar scaffolds in some of the early proposals?
^^^^^^^ The molecules they propose really remind me of Leflunomide - have we been able to crystalize teriflunomide with mpro? @AnthonyA
I suggested this many a moon ago but so far no action.
https://covid.postera.ai/covid/submissions/bab12148-3b63-4d08-9c86-25e56d7a9288
Looking for funding to do this. the problem is that it’s an imuno suppressant so not the best thing to dose in critical care but an opportunity i) to see if it’s a warhead (or its prodrug in vivo/vitro), ii) if it has Mpro activity, iii) if it then can be reverse engineered (SOSA) and the immunosup. removed and the side affinity (MPro) amplified… An interesting project I’ve put forward but waiting a response!!!
@JSPEN - fell through the filters so far.
2 reasons why it probably wasn’t prioritised
MW 202, which means it might have have just been removed when we were looking at drug size compounds
(too) simple structures, hard to think that it would be specific.
Of course it is an interesting experiment to do but moonshot was to get inhibition quickly and original thinking was that we had to be in the drug like space. We are often reviewing priorities though. Get Storm to sneak in the lab and send it to us 
We have it in the lab on another project but can’t go in yet. This will happen!
You can buy it from Tocris also!
Ghosh et al, ‘Drug Development and Medicinal Chemistry Efforts Toward SARS‐Coronavirus and Covid‐19 Therapeutics’ appeared in ChemMedChem a couple of days ago: https://doi.org/10.1002/cmdc.202000223
Might be worth checking the pKa of JOH-SUS-bab-1. I would guess that its anionic at physiological pH.