Topic automatically created for discussing the designs at:
https://covid.postera.ai/covid/submissions/RUB-POS-1325a9ea
RUB-POS-1325a9ea-12 looks like an interesting compound and I’ll mention @ruby who designed it. RUB-POS-1325a9ea-12 (IC50: 2.1 μM) is about twofold more potent than the naphthyridine MAT-POS-f7918075-5 (IC50: 4.3 μM) and I would expect N-methylation of pyrazolopyridine (RUB-POS-1325a9ea-14) to reduce the energetic penalty for adoption of the bound conformation.
Aza substitution of aromatic rings is typically beneficial for ADME. In particular, aza-substitution increases polarity (good for solubility and reduces affinity for anti-targets) and reduces the availability of the π electrons which might be expected to improve metabolic stability. Typically, aza-substitution reduces affinity for the primary target and a well-tolerated aromatic nitrogen should always be seen as an oportunity. I’ll mention @mc-robinson @edgriffen @alphalee since I understand that they are currently looking at ways to increase the metabolic stability for isoquinolines. The PDB associated with the PET-UNK-e274cad4 includes binding modes proposed for RUB-POS-1325a9ea-14 and the 1-methyl analog of the azaindole JIN-POS-6dc588a4-14 designed by @jin
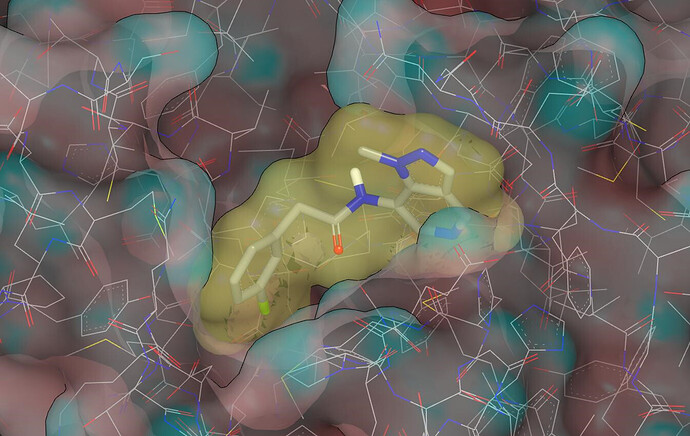
Here’s a graphic showing the binding mode proposed for RUB-POS-1325a9ea-14 (molecular surface of the protein has been colored by curvature):