Topic automatically created for discussing the designs at:
https://covid.postera.ai/covid/submissions/PET-UNK-d9de6a0b
Thanks for these Pete, we have a real synthetic problem alkylating the tertiary alcohol - currently our best way of functionalising it (other than methyl) is to use ethylene oxide, and then alkylate the extended OCH2CH2OH to give OCH2CH2OR. So if you have ideas that could use that - would be interesting.
Is it possible to alkylate that alcohol with allyl or propargyl bromide, for example? These groups could be transformed into something else later: hydrogenation, oxidative cleavage, Suzuki/Sonogashira coupling, cyclopropanation, 3+2 with azides, thiol-ene and more (I think that introducing benzyl or cyclopropylmethyl could also be possible and perhaps useful)
Thanks for the info, Ed, and I’d previously submitted PET-UNK-3bb57da2-3 and PET-UNK-3bb57da2-4 (respectively the O-methyl and O-cyclopropyl analogs of ED_-GRI-5b13fbe2-72). I’ve just submitted the O-ethyl analog as PET-UNK-e5587e5d-1. The pdb file associated with this submission has predicted binding modes for ED_-GRI-5b13fbe2-72, PET-UNK-3bb57da2-3, PET-UNK-3bb57da2-4 and the single design PET-UNK-e5587e5d-1 from the submission.
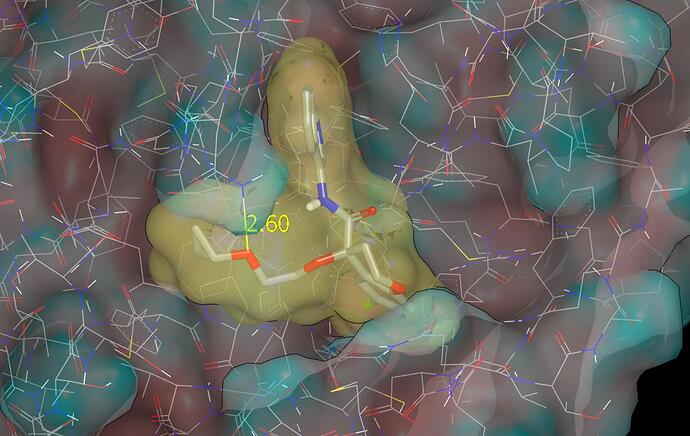
My view is that PET-UNK-3bb57da2-3 is essentially a no-brainer while I believe that PET-UNK-3bb57da2-4 will make better contact with the molecular surface of the protein. The following graphic shows the proposed binding mode for PET-UNK-3bb57da2-4. While the contact between the ether oxygen and the protein is somewhat unconvincing, at least the ether oxygen is not forced against a non-polar region of protein molecular surface.