Topic automatically created for discussing the designs at:
https://covid.postera.ai/covid/submissions/PET-UNK-7fb4f80a
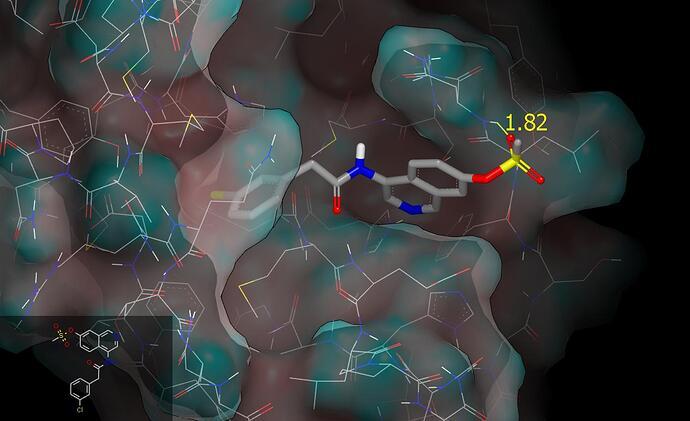
Hi @mc-robinson @edgriffen You may wish to methylsulfonyloxy as a substituent with a view to protecting the P1 isoquinoline from metabolism. When placed at C7 one of the sulfonyl oxygen atoms can accept a hydrogen bond from the backbone amide NH of N142. Although alkylsulfonates would usually be regarded as unacceptable on the grounds of being alkylating agents, this is not a problem for arylsulfonates (the substructure is found in the molecular structures of the investigational drugs Tesaglitazar and Ladarixin) which I would anticipate to be more stable than the corresponding esters. The conformation with sulfur out of the plane of the aromatic ring is also observed in CSD structures. Here is a graphic showing the proposed binding mode for PET-UNK-7fb4f80a-1.