Topic automatically created for discussing the designs at:
https://covid.postera.ai/covid/submissions/PET-UNK-7d68125a
The objective of the designs in this submission is to present the nitrile warhead to the catalytic cysteine in a manner that is compatible with the Ugi synthesis. The designs are derived from LON-WEI-2e27a2e5-1 | MAT-POS-f2460aef-1 | JOE-NOR-4e4adc6b-1 and so I’ll mention @londonir @mc-robinson @Joe. I’ll also include @Daren_Fearon @JSPEN with whom I’ve discussed covalent inhibitors and @AnthonyA who is interested in covalent inhibitors.
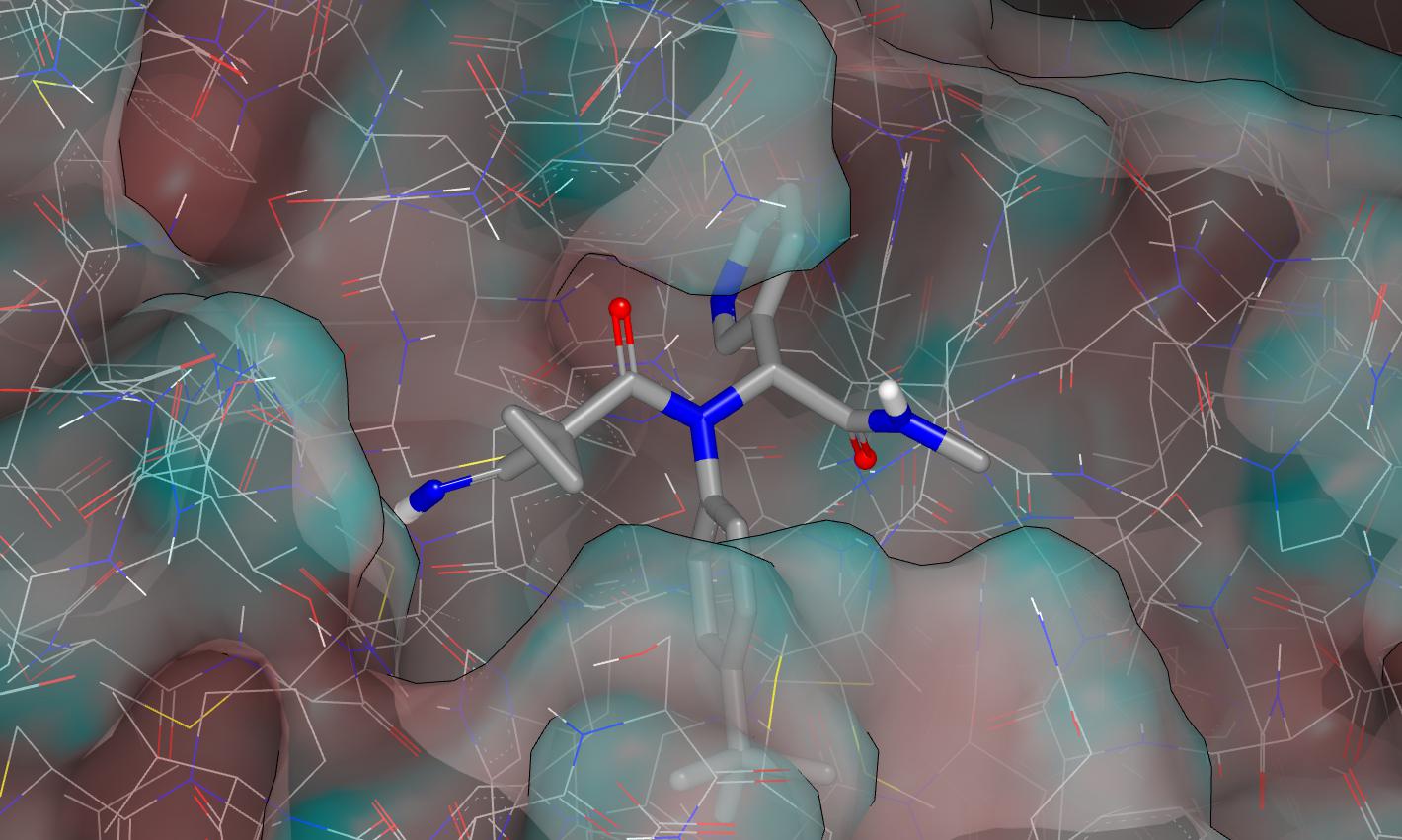
I’ve used pdb:6w63 for modelling and truncated the fluorophenethyl group to methyl. Here is a graphic (surface colored by curvature) showing the proposed binding mode for PET-UNK-7d68125a-1.
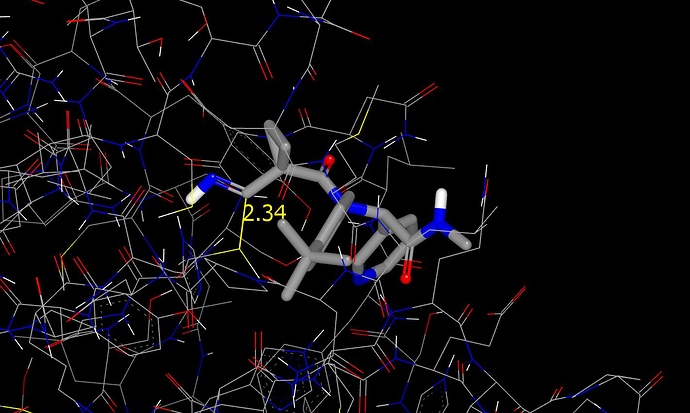
As stated in the submission notes, the designs were first modeled as their des-cyano analogs and the imine substructure was subsequently edited into each structure. This figure shows how the procedure places the imine carbon at close to bonding distance from the catalytic cysteine thol.
Of the designs, PET-UNK-7d68125a-4 (with methylene linking the warhead to the rest of the molecule) is likely to be the easiest to synthesize. However, I would be concerned about the potential of this design to enolize and/or to form a carbanion.