Topic automatically created for discussing the designs at:
https://covid.postera.ai/covid/submissions/MIC-UNK-4e776895
I’ve just noticed that isoxazoles could be possibly made through convergent route 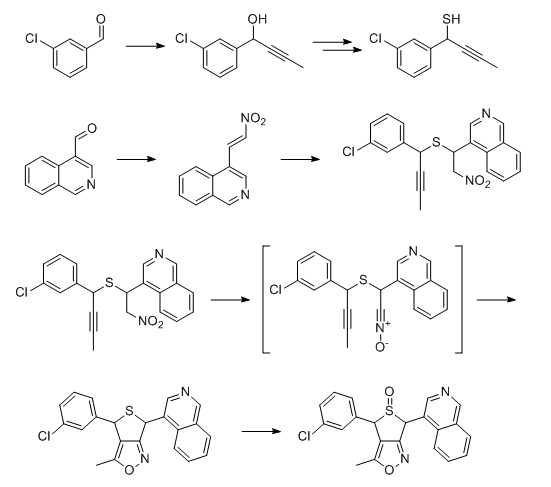
At worst pyrazole would need some kind of protecting group. I know that it generally should be possible to perform asymmetric organocatalytic thiol addition to nitroalkene, such as in dx.doi.org/10.1039/C2CC17965B, and I suspect that something similiar could be done for alkyne addition though probably with some metal complex as catalyst, and then there are chiral sulfide oxidation procedures so I guess entire thing could be made as a single isomer when it would be clear which it is. Probably the most challenging step would be intramolecular nitrile oxide cycloaddition. Synthesis of all 8 isomers as a mixture should be even easier.