Topic automatically created for discussing the designs at:
https://covid.postera.ai/covid/submissions/JAR-IMP-f500b4ba
I’m very interested to know what any medicinal chemists think of these fused macrocycle cores, which the scoring algorithm believe are chemical similar to the central groups of Boceprevir.
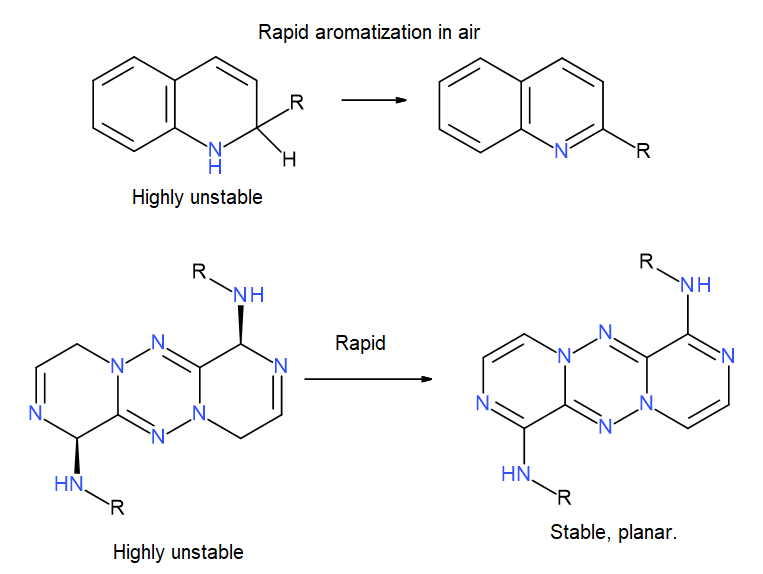
It’s an interesting design concept, but unfortunately these compounds would be incredibly unlikely to be stable, they are just waiting to oxidise / aromatize into fully conjugated tricyclic aromatics  . (as a simpler analogy, look into the stability of diydroquinolines):
. (as a simpler analogy, look into the stability of diydroquinolines):
Although a bit of a faff, what about using a C-F (rather than C-H) to stabilise the non planar structure? Or, other bioissosteres?
C-Me would be easier synthetically than C-F; however we’d still most likely be looking at some incredibly tricky synthesis… could be interetsing to see what the postera AI makes of synthesizing such molecules - perhaps add in a couple of new submissions with C-Me and C-F swapped in to stabilize the conformation and then ask someone at postera e.g. @mc-robinson to predict synthetic routes.
The other option would be to see if any of the suggestion made via your initial approach have a core cyclic system which is not aromatic (boceprevir core is not aromatic)
I agree these structures will likely be unstable, at first I also thought rearomatization by oxidation would be the problem, but the tricyclic system is anti-aromatic and will not be formed imo (not planar nor stable). The central [1,4-Dihydro-1,2,4,5-tetrazines] core is also antiaromatic, but examples of such structures with four alkyl groups in the same position is described in literature according to scifinder. (Chemische Berichte, 116(6), 2261-74; 1983, Neugebauer, Franz Alfred)
A synthesis of these compounds will be very hard (but maybe not impossible) and for this reason imo not a viable option for the covid moonshot project.
I like the submissions by the computational chemists and I’m jealous, that I’m not skilled to use such software. But unfortunately a lot of the submissions contain very unstable fragments such as cyclopentadienes, anti- or non-aromatic unsaturated heterocycles in strange tautomeric forms, labile functional groups and very apolar large aromatic ring systems.
Indeed you are correct…my bad . So perhaps they would not be that inclinedto aromatize… but as you say the core itself is not aromatic either, so not sure how stable it would be.
If it were stable that would just leave us with the tricky issue of how to synthesize…
I’m sure a lot of stuff will be triaged out manually or by computer, e.g. NCH2N, I saw in a few comments earlier.
I’d still urge non-chemists etc to suggest “out of the blue” structures. Even if the proposed structure is not synthetically tractable, it will stir up some interest and the community will no doubt find a way around it by e.g… bioisosteric replacement, conformational restriction etc… I’m sure we will get some chemical space predictions that are quite different to some of our clumped together thoughts!
I totally agree, collaboration is the only solution to both problems.
I agree wit @JHullaert that the tricyclic 1,2,4,5-tetrazine system at the bottom left of @Ben_DNDi’s snapshot seems to be counterintuitive, for two reasons:
-
If fully planar, one of the nitrogens (nitrogen 5) will be very strained, as there doesn’t seem to be space for the lone pair. Moreover, the lone pair won’t be able to delocalise into the whole conjugated ring system either.
-
As @JHullaert pointed out, it seems that the ring system will be antiaromatic (4n electrons). So counterintuitively, it might be better for the molecule to adopt a non-planar shape.
Out of curiosity, how common is this scaffold found in the literature? I did a Reaxys molecular search as well as a nomenclature Google / Google Scholar search, but nothing turned up.
There’s a lovely pertinent review on unusual ring systems that might be worth a read:
https://pubs.acs.org/doi/10.1021/jm801513z
Heteroaromatic Rings of the Future
-
William R. Pitt*
, * David M. Parry†
, * Benjamin G. Perry‡
, and * Colin R. Groom§
View Author Information
Cite this: J. Med. Chem. 2009, 52, 9, 2952–2963
Hmm. One of those author names looks quite familiar 
Thank you so much for this, @JSPEN! It is indeed a good overview for heterocycles.
Using Scopus, I found a 2019 review that could be seen as an “update” for this review.
Link below if anyone else is interested:
https://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-0037-1611279
Also, what I found especially interesting during my search, is that nobody seems to have explored the implications of these heterocycles in the context of the pharmaceutical industry.
Questions like:
-
How might these molecules containing these heterocycles behave in the binding site? would they be planar or would they pucker to avoid antiaromaticity? Would they tautomerise to achieve aromaticity?
-
Related to the first point above–how would these considerations affect the docking scores and the related penalties associated with these docking scores? For example, Schrodinger’s Maestro sometimes has computational penalties that are included in the computation of docking scores.
-
Lastly, from a more practical perspective–the stability of molecules may also impact storage, a consideration which @Ben_DNDi has already elaborated on above!