Topic automatically created for discussing the designs at:
https://covid.postera.ai/covid/submissions/EDJ-MED-fed7ac0b
Discussion by email from Bobby Glen: " > Many of the compounds are close enough to the Cys145 to make a close S-…NH (from the amide) contact. The hydrogen bonds between S- and NH are stronger than for the equivalent ketone, and are mostly dispersion, so angular dependence is not such an issue. The neutron mPRO study suggests that the pre-transition state is S-minus. The catalytic dyad was observed in a zwitterionic form in the enzyme without substrate or an inhibitor. The zwitterion comprises a deprotonated, negatively charged Cys145 thiolate and a doubly protonated, positively charged His41 imidazolium situated ∼3.8 Å apart from each other.
Which suggests, if synthesisable, to try and get the S…HN contact back, this, and related analogs may be a possibility. 3-Pyrazolidinone exists. Not sure how to get it into a spiro fusion though. Compound is improved for hydrophilicity, but would still need to block the other amide hydrolysis and de-methylation."
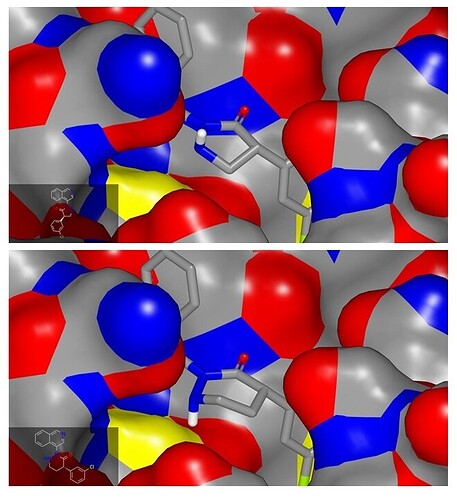
Hi @edgriffen @RGlen, this looks like an interesting idea although I would advise using a 6-membered ring rather than a 5-membered ring to test it. Hydrazine derivatives tend adopt conformations that minimize the π interaction between the nitrogens which means that the NH is likely to be axial (this effect is illustrated by the SEVYEY CSD structure). Assuming that the aza-analog of PET-UNK-c9c1e0d8-4 (MPro-P0010 crystal structure) puckers in an analogous manner to the parent compound, the NH will be oriented toward solvent rather than toward the catalytic cysteine. Similarly reasoning suggests that the NH of the aza-analog of PET-UNK-c9c1e0d8-3 (MPro-P0019 crystal structure) will be directed toward the catalytic cysteine.
I would not expect spiro-fusion to affect puckering of the lactam rings (I know that a crystal structure for EDJ-MED-8bb691af-4 is available as Mpro-P2222 but I can’t currently see the crystal structures in Fragalysis).
Hi @edgriffen @RGlen, I’ve followed this up with the PET-UNK-7b413b46 submission. Top image in graphic below shows non-spirocyclic analog derived from your submission (NH pointing away from catalytic cysteine) and its 6-membered analog (NH pointed toward catalytic cysteine). The conformational preferences reflect the pucker of the ring (determined by the need to keep 3-chlorophenyl equatorial) and the tendency of hydrazine lone pairs to orient orthogonally (alpha effect). While I would not anticipate significant gains in potency to result from the interaction between NH and catalytic cysteine, the NH would be a good synthetic handle for accessing the oxyanion hole (5-membered ring) or catalytic cysteine (e.g. N-cyano analog of 6-membered ring).