Topic automatically created for discussing the designs at:
https://covid.postera.ai/covid/submissions/EDJ-MED-50011917
Hi Ed @edgriffen
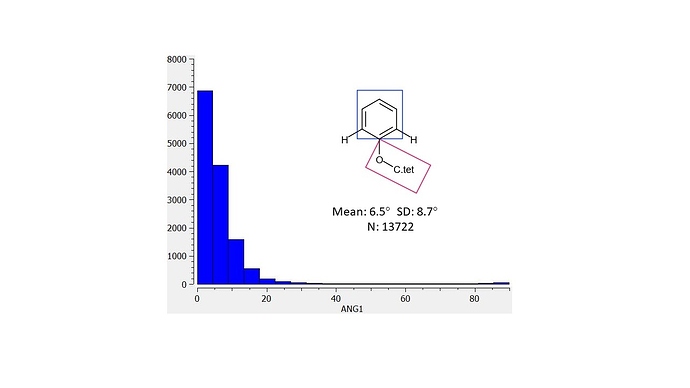
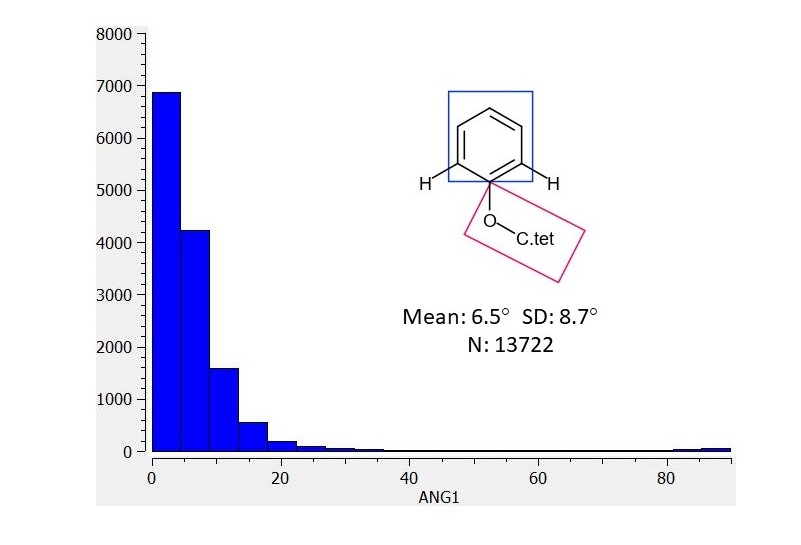
I’ll mention @JohnChodera since we briefly discussed this on twitter and @mc-robinson in case this type of information is of general interest for design. I’ve looked at distributions in the CSD for structures for ‘anisole’ torsions (phenol O-substituted with tetrahedral carbon). I defined 2 planes (shown as rectangles in the graphics) and the histogram is for the angle between the planes). In the first analysis the ‘anisolic’ oxygen is flanked by two CHs and the dearth of 90 degree angles should be apparent.
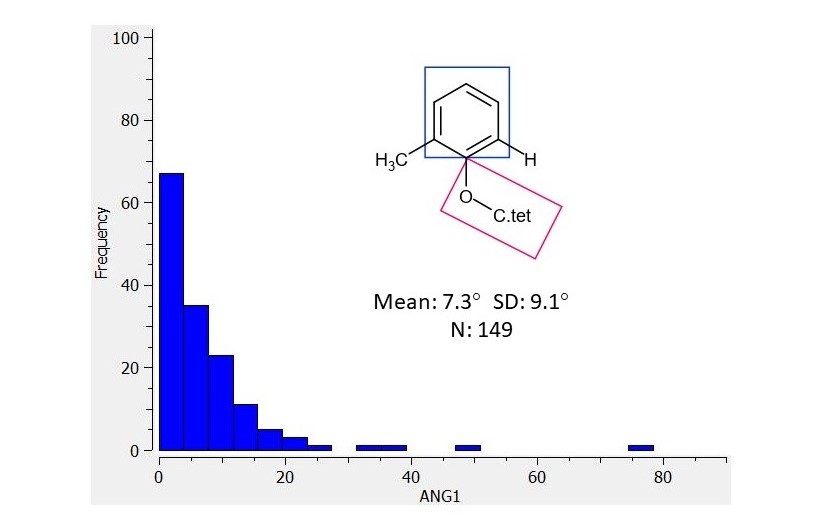
In the second analysis the ‘anisolic’ oxygen is flanked by a methyl and a CH. This shows that a flanking methyl has minimal influence on the torsion distribution and does not force a 90 degree torsion.
The beta-lactam does look to me like an accident waiting to happen (happy to be proven wrong) and I’d also be worried about chemical stability. Nevertheless, given weaknesses in the binding mode (likely torsional strain; ‘unmatched’ carbonyl oxygen), its contribution to affinity is striking. This article on covalent warhead reactivity features some beta-lactams and may be of interest if you’ve not already seen it.
Hi @pwkenny,
The beta-lactam does look to me like an accident waiting to happen (happy to be proven wrong) and I’d also be worried about chemical stability.
This is why the first Folding@home sprint focused on replacing the beta-lactam by exhaustively considering all possible replacements Enamine could synthesize from a common intermediate using two robust reaction routes.
You can find the compounds we ordered as a result of this here:
- Buchwald : MAT-POS-044491d2
- Suzuki : MAT-POS-f42f3716
In future, it would help us if you started these threads with a clear statement of
- Which specific compound(s) you’re discussing, using the PostEra IDs so we understand what you’re talking about
- A clear articulation of what you think the issues are
- A proposed solution, if you have one in mind
Reading your note a few times, I’m having a difficult time synthesizing any of these three essential elements from what you’ve written.
Best,
John
Hi John @JohnChodera
Apologies for the lack of clarity and I will endeavour to follow your directives in future communications. Probably best to start with the rationale presented by Ed @edgriffen for the three designs:
“O linked lactams with ortho substituent designs to stabilised the out of plane conformation as seen in crystal structure Mpro-10789. C linked lactams designed to prefer out of plane conformation and to be more metabolically stable.”
I found this rationale to be clear. Ed recognizes the likely torsional strain as a ‘weakness’ in the binding mode and has proposes solutions (replace linking O with CH2: EDJ-MED-50011917-1 | place methyl ortho to linking O: EDJ-MED-50011917-2 ). The first CSD plot shows the distribution for the angle between the planes of the ring and an ‘anisolic’ group (oxygen linking tetrahedral carbon to aromatic ring) that is flanked by two CHs. It is this coplanar conformational preference that Ed seeks to perturb in his designs.
My interpretation of the second CSD plot is that placing a methyl ortho to the ‘anisolic’ group does not appear to push the ‘anisolic’ group into an out-of-plane conformation. As such, I would suggest that EDJ-MED-50011917-2 should be seen as a lower priority than EDJ-MED-50011917-1.
I see explicit targeting the backbone amide carbonyl of E166 as a useful design tactic since interactions with this hydrogen bond acceptor have observed in crystal structures of both fragments and peptidomimetics. One direction in which the EDJ-MED-50011917-1 design might be taken would be to reduce the beta lactam to azetidine (presents a cationic hydrogen bond donor to E166). The pKa of this azetidine could probably be brought to below 7 by difluorination of the benzylic carbon (Merck Frosst scientists used fluorine in an analogous manner in the design of the cathepsin K inhibitor odanacatib). I have also proposed PET-UNK-372a2f82 which replaces the beta lactam of EDJ-MED-50011917-1 with pyrazole.
@pwkenny : Thanks for the clear, lucid explanation here! This is super helpful and at provides a number of promising paths to follow up on!
~ John
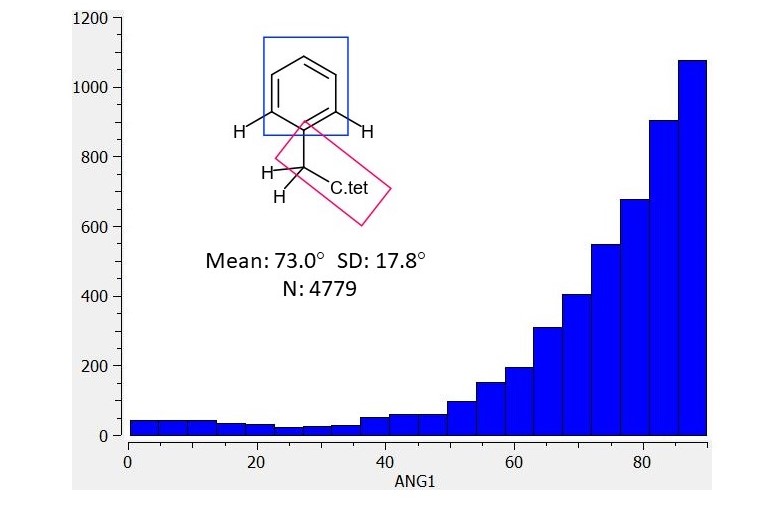
Hi @pwkenny - Thanks for the detailed analysis Pete. Do you have the equivalent torsion plots for the CH2 linked beta lactam (on the “change one thing at a time” strategy). I’m interested to see if EDJ-MED-50011917-1 shows more of an out of plane bias than the O linked current lead. As @JohnChodera said - we’re currently working hard to get away from the beta-lactam if we can. It’s a it seems like a flexible region if you examine the crystal structures in that region.
Hi Ed @edgriffen , here is the corresponding histogram for benzene ring linked to tetrahedral carbon by a methylene group (flanked by CHs) and I think that your EDJ-MED-50011917-1 design would be well worth synthesizing
I certainly agree with John @JohnChodera that this region of the binding site is flexible and the pyrazole design and I believe that the PET-UNK-372a2f82 pyrazole design will need this flexibility in order to donate the hydrogen bond to E166. Something to consider would be generation of a small set of structures with the potential to form this hydrogen bond that could be evaluated by simulation (e.g. does the hydrogen bond form?). I would guess that we would be looking at no more than 10-20 structures in the first instance and I can provide more detail if this would be of interest.
Thanks Pete, we can push prioritising the C linked analogues up the synthesis queue - in particular finding how to do the benzylic alkylation. The O linked series are straightforward to make from the phenol and the OAc beta lactam.
Hi Ed, I think that it’d be worth trying to reduce the beta lactam to azetidine for the O-linked species since the proximity of oxygen to nitrogen will keep pKa well below 7. Could be a relatively easy way to map some SAR for this part of the molecular structure although I’d still be concerned about having two heteroatoms bonded to sp3 carbon (especially given ring strain). I’ll submit as a design and then link our discussion.