I’ll mention @alphalee @frankvondelft @JohnChodera @londonir @mc-robinson @edgriffen @RGlen in case this material is of interest.
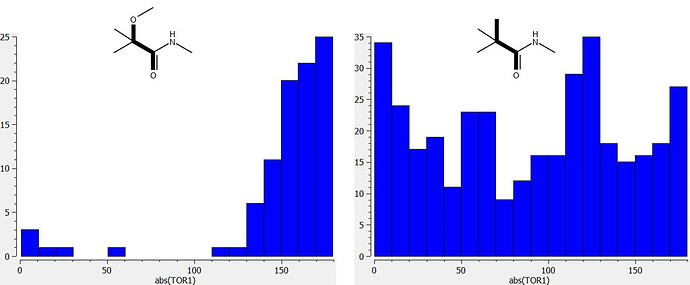
I’ve run a couple of CSD searches in order to better understand the conformational preferences of the methoxy substituent of PET-UNK-29afea89-2. The dihedral angle profiles shown in the graphic below indicate that the oxygen of the methoxy group tends to eclipse the amide NH. Although I would not regard interaction between methoxy oxygen and NH as a proper hydrogen bond, the interaction has the potential to prevent the amide NH as a hydrogen bond donor (one can think in terms of a secondary electrostatic interaction between the methoxy oxygen and any hydrogen bond acceptor that the amide NH happens to interact with). I speculate that this may be the basis for any benefits of the methoxy with respect to translation of enzyme inhibition to activity in cell-based assays (maybe the amide NH is recognized by a transporter?).
Here are the dihedral angle distributions (the bonds that define each dihedral are constrained to be acyclic)