The following from the first 160 submissions are all already contained in Enamine Real space, we should consider starting by ordering some/all of these soon.
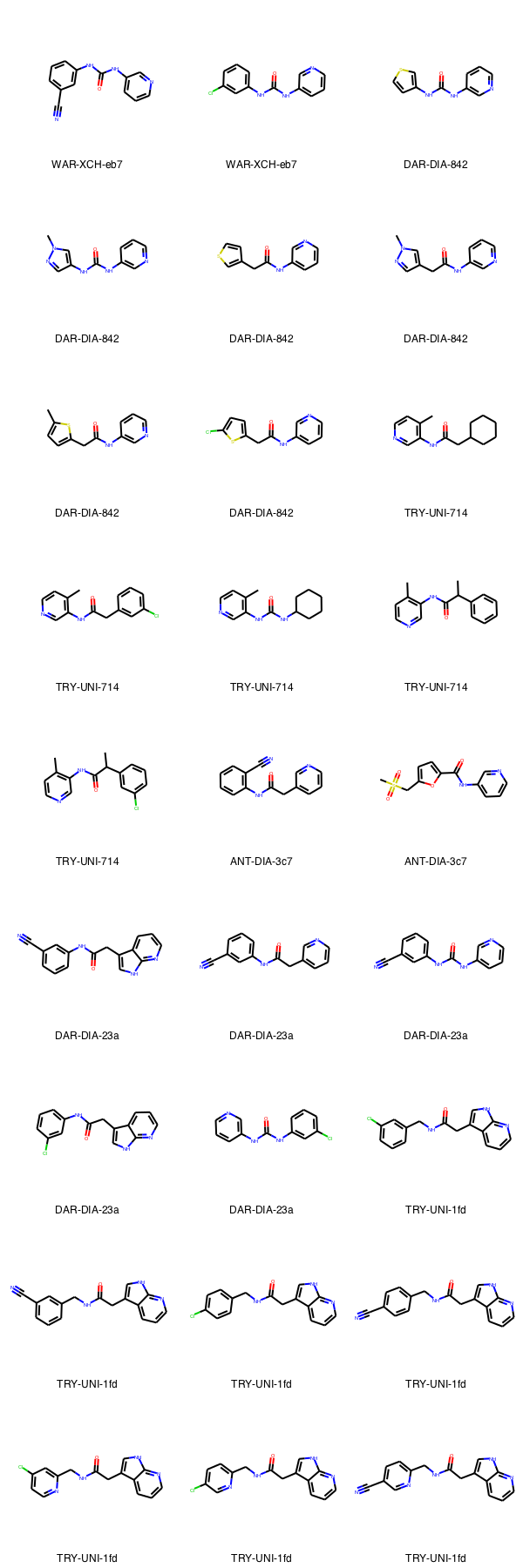
The following from the first 160 submissions are all already contained in Enamine Real space, we should consider starting by ordering some/all of these soon.

Can you have them as a workable sheets with smiles? I don’t see any reasons why we should not already send them the list
Yes. Let me just rerun right now and include if the compound is in the screening compounds. One of the submissions today ( PAT-UNK-b2d-1 , C1=C(C#N)C=C(CN2CCN(C(=O)C)CC2)C=C1) is actually in stock in the screening library. Will comment on it in a separate thread.
Ok here are all of the ones that look immediately ready to go. They are either available as building blocks, screening compounds, or in the real space. Will follow tomorrow with comments on how we can get at some of the more complex designs.
| SMILES | CID | real_space | SCR | BB | |
|---|---|---|---|---|---|
| 14 | N#Cc2cccc(NC(=O)Nc1cccnc1)c2 | WAR-XCH-eb7 | Z195739680 | Z195739680 | False |
| 19 | Cc2cccc(NC(=O)Nc1cccnc1)c2 | WAR-XCH-eb7 | False | Z44592351 | False |
| 20 | O=C(Nc1cccnc1)Nc2cccc(Cl)c2 | WAR-XCH-eb7 | Z44592325 | False | False |
| 22 | C1=CC=NC=C1CCNS©(=O)=O | ANT-DIA-b7f | False | Z300622420 | False |
| 25 | C1C(CCNC(=O)C)=CC=CC=1 | ANT-DIA-b7f | False | False | BBV-39132223 |
| 26 | C1(N=CSC=1)CCNS(=O)©=O | ANT-DIA-b7f | False | False | BBV-39159100 |
| 43 | C1=C(C#N)C=C(CN2CCN(C(=O)C)CC2)C=C1 | PAT-UNK-b2d | Z46180946 | Z46180946 | False |
| 48 | O=C(NC1C=NC=CC=1)NC1C=CSC=1 | DAR-DIA-842 | Z1430585289 | Z1430585289 | False |
| 49 | O=C(NC1C=NC=CC=1)NC1C=NN©C=1 | DAR-DIA-842 | Z603563998 | False | FCH9186936 |
| 57 | O=C(CC1=CSC=C1)NC1C=CC=NC=1 | DAR-DIA-842 | Z31791777 | False | False |
| 58 | O=C(NC1C=NC=CC=1)CC1C=NN©C=1 | DAR-DIA-842 | Z993021208 | False | False |
| 59 | O=C(NC1C=NC=CC=1)CC1=CC=C©S1 | DAR-DIA-842 | Z815312198 | False | False |
| 60 | O=C(CC1SC(Cl)=CC=1)NC1C=CC=NC=1 | DAR-DIA-842 | Z2010253653 | False | False |
| 75 | O=C(CC1CCCCC1)NC1C©=CC=NC=1 | TRY-UNI-714 | Z1129284100 | False | False |
| 77 | O=C(CC1C=C(Cl)C=CC=1)NC1C©=CC=NC=1 | TRY-UNI-714 | Z1129289650 | False | False |
| 81 | O=C(NC1CCCCC1)NC1C©=CC=NC=1 | TRY-UNI-714 | Z1531420463 | False | False |
| 88 | O=C(C(C1C=CC=CC=1)C)NC1C©=CC=NC=1 | TRY-UNI-714 | Z1724389531 | False | False |
| 89 | O=C(C(C1C=C(Cl)C=CC=1)C)NC1C©=CC=NC=1 | TRY-UNI-714 | Z1264525706 | False | False |
| 120 | O=C(CC1C=CC=NC=1)NC1C(C#N)=CC=CC=1 | ANT-DIA-3c7 | Z1172500933 | False | False |
| 122 | O=C(NC1C=CC=NC=1)C1OC(CS(=O)©=O)=CC=1 | ANT-DIA-3c7 | Z995832158 | False | False |
| 128 | O=C(NC1C=C(C#N)C=CC=1)CC1C2C(=NC=CC=2)NC=1 | DAR-DIA-23a | Z1400784309 | False | False |
| 131 | O=C(CC1C=CC=NC=1)NC1C=CC=C(C#N)C=1 | DAR-DIA-23a | Z1171321476 | False | False |
| 132 | O=C(NC1C=CC=NC=1)NC1C=CC=C(C#N)C=1 | DAR-DIA-23a | Z195739680 | Z195739680 | False |
| 139 | O=C(CC1=CNC2=NC=CC=C12)NC1C=CC=C(Cl)C=1 | DAR-DIA-23a | Z1400781098 | False | False |
| 141 | O=C(NC1C=C(Cl)C=CC=1)NC1C=NC=CC=1 | DAR-DIA-23a | Z44592325 | False | False |
| 152 | O=C(CC1C2C=CC=NC=2NC=1)NCC1C=C(Cl)C=CC=1 | TRY-UNI-1fd | Z1400783129 | False | False |
| 153 | O=C(CC1C2C=CC=NC=2NC=1)NCC1C=C(C#N)C=CC=1 | TRY-UNI-1fd | Z1400838108 | False | False |
| 154 | O=C(CC1C2C=CC=NC=2NC=1)NCC1C=CC(Cl)=CC=1 | TRY-UNI-1fd | Z1400780364 | False | False |
| 155 | O=C(CC1C2C=CC=NC=2NC=1)NCC1C=CC(C#N)=CC=1 | TRY-UNI-1fd | Z1400838206 | False | False |
| 156 | O=C(CC1C2C=CC=NC=2NC=1)NCC1C=C(Cl)C=CN=1 | TRY-UNI-1fd | Z1575152133 | False | False |
| 158 | O=C(CC1C2C=CC=NC=2NC=1)NCC1C=CC(Cl)=CN=1 | TRY-UNI-1fd | Z1593808911 | False | False |
| 159 | O=C(CC1C2C=CC=NC=2NC=1)NCC1C=CC(C#N)=CN=1 | TRY-UNI-1fd | Z1767447079 | False | False |
@ AnthonyA do you have a list of all the fragments you used (including unsuccessful) somewhere, so we can make sure nothing we are looking to order has already been tried unsuccessfully.
@mc-robinson: yes we do, it is a csv file on the website. I will send it to you with details so you understand what it means. However we should take into account the fact that more compounds will be screened (when shutdown is finished) and that it should be pulled from restful api instead, or a system where you can regularly check the new experiments. Will get back to you on this
Thanks, and yes an API would be great. Or I could even draw the data from a live google sheet, as it is updated, if need be
@AnthonyA, here are the purchase-able compounds that were designed building off of at least one covalent fragment
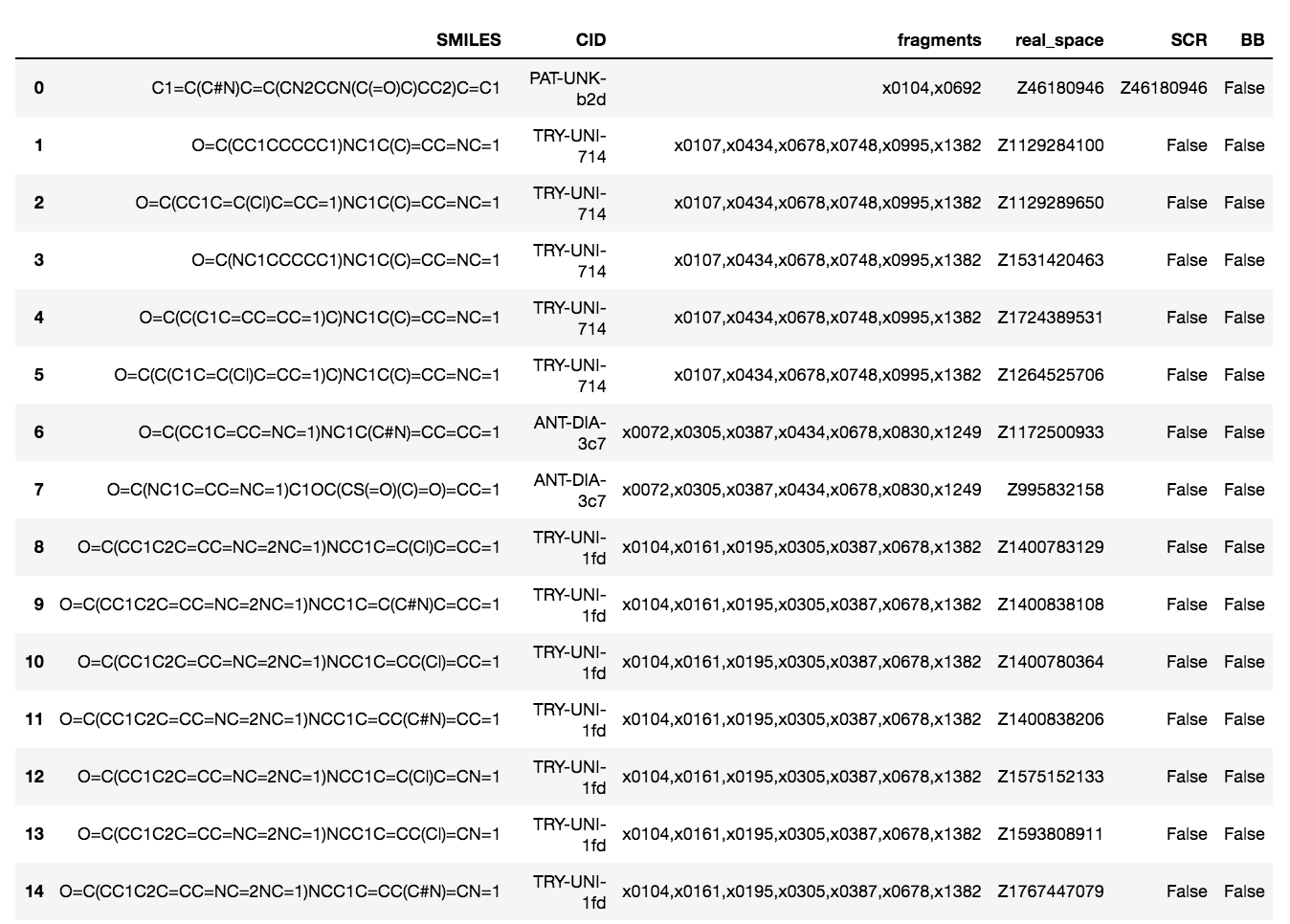
List of SMILES here:
['C1=C(C#N)C=C(CN2CCN(C(=O)C)CC2)C=C1',
'O=C(CC1CCCCC1)NC1C(C)=CC=NC=1',
'O=C(CC1C=C(Cl)C=CC=1)NC1C(C)=CC=NC=1',
'O=C(NC1CCCCC1)NC1C(C)=CC=NC=1',
'O=C(C(C1C=CC=CC=1)C)NC1C(C)=CC=NC=1',
'O=C(C(C1C=C(Cl)C=CC=1)C)NC1C(C)=CC=NC=1',
'O=C(CC1C=CC=NC=1)NC1C(C#N)=CC=CC=1',
'O=C(NC1C=CC=NC=1)C1OC(CS(=O)(C)=O)=CC=1',
'O=C(CC1C2C=CC=NC=2NC=1)NCC1C=C(Cl)C=CC=1',
'O=C(CC1C2C=CC=NC=2NC=1)NCC1C=C(C#N)C=CC=1',
'O=C(CC1C2C=CC=NC=2NC=1)NCC1C=CC(Cl)=CC=1',
'O=C(CC1C2C=CC=NC=2NC=1)NCC1C=CC(C#N)=CC=1',
'O=C(CC1C2C=CC=NC=2NC=1)NCC1C=C(Cl)C=CN=1',
'O=C(CC1C2C=CC=NC=2NC=1)NCC1C=CC(Cl)=CN=1',
'O=C(CC1C2C=CC=NC=2NC=1)NCC1C=CC(C#N)=CN=1']
And an image here
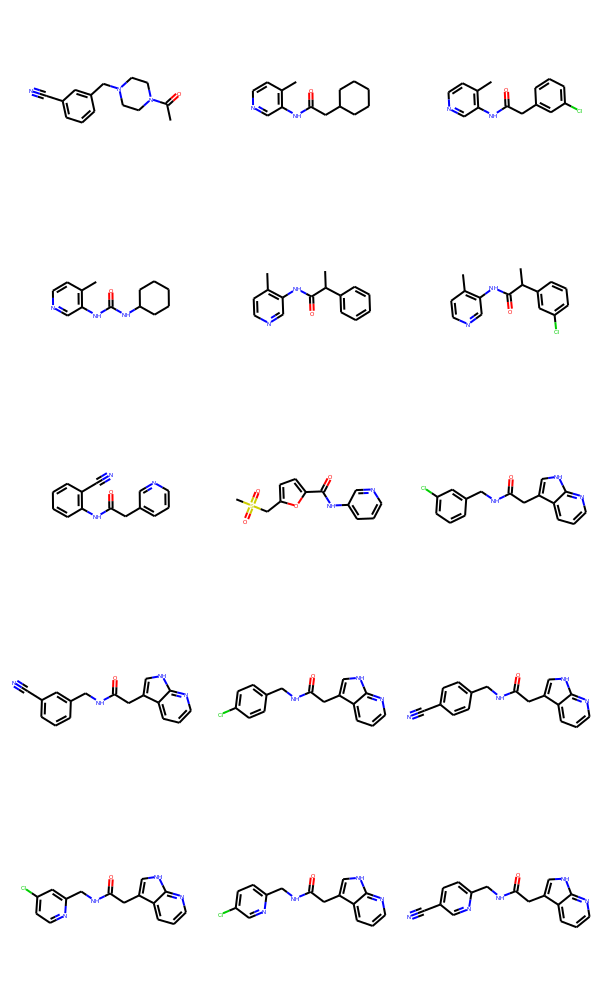
Hi, I am updating this after some new submissions.
SMILES CID real_space SCR BB covalent_frag
0 N#Cc2cccc(NC(=O)Nc1cccnc1)c2 WAR-XCH-eb7 Z195739680 Z195739680 False False
1 Cc2cccc(NC(=O)Nc1cccnc1)c2 WAR-XCH-eb7 False Z44592351 False False
2 O=C(Nc1cccnc1)Nc2cccc(Cl)c2 WAR-XCH-eb7 Z44592325 False False False
3 C1=CC=NC=C1CCNS(C)(=O)=O ANT-DIA-b7f False Z300622420 False False
4 C1C(CCNC(=O)C)=CC=CC=1 ANT-DIA-b7f False False BBV-39132223 False
5 C1(N=CSC=1)CCNS(=O)(C)=O ANT-DIA-b7f False False BBV-39159100 False
6 C1=C(C#N)C=C(CN2CCN(C(=O)C)CC2)C=C1 PAT-UNK-b2d Z46180946 Z46180946 False True
7 O=C(NC1C=NC=CC=1)NC1C=CSC=1 DAR-DIA-842 Z1430585289 Z1430585289 False False
8 O=C(NC1C=NC=CC=1)NC1C=NN(C)C=1 DAR-DIA-842 Z603563998 False FCH9186936 False
9 O=C(CC1=CSC=C1)NC1C=CC=NC=1 DAR-DIA-842 Z31791777 False False False
10 O=C(NC1C=NC=CC=1)CC1C=NN(C)C=1 DAR-DIA-842 Z993021208 False False False
11 O=C(NC1C=NC=CC=1)CC1=CC=C(C)S1 DAR-DIA-842 Z815312198 False False False
12 O=C(CC1SC(Cl)=CC=1)NC1C=CC=NC=1 DAR-DIA-842 Z2010253653 False False False
13 O=C(CC1CCCCC1)NC1C(C)=CC=NC=1 TRY-UNI-714 Z1129284100 False False True
14 O=C(CC1C=C(Cl)C=CC=1)NC1C(C)=CC=NC=1 TRY-UNI-714 Z1129289650 False False True
15 O=C(NC1CCCCC1)NC1C(C)=CC=NC=1 TRY-UNI-714 Z1531420463 False False True
16 O=C(C(C1C=CC=CC=1)C)NC1C(C)=CC=NC=1 TRY-UNI-714 Z1724389531 False False True
17 O=C(C(C1C=C(Cl)C=CC=1)C)NC1C(C)=CC=NC=1 TRY-UNI-714 Z1264525706 False False True
18 O=C(CC1C=CC=NC=1)NC1C(C#N)=CC=CC=1 ANT-DIA-3c7 Z1172500933 False False True
19 O=C(NC1C=CC=NC=1)C1OC(CS(=O)(C)=O)=CC=1 ANT-DIA-3c7 Z995832158 False False True
20 O=C(NC1C=NC=CC=1)NC1C=CC=C(Cl)C=1 JOR-UNI-2fc Z44592325 False False False
21 C1CN(C(=O)C)CCN1CC1=CC(O)=CC(Cl)=C1 KIM-UNI-2ee Z2967471722 False False True
22 O=C(NC1C=C(C#N)C=CC=1)CC1C2C(=NC=CC=2)NC=1 DAR-DIA-23a Z1400784309 False False False
23 O=C(CC1C=CC=NC=1)NC1C=CC=C(C#N)C=1 DAR-DIA-23a Z1171321476 False False False
24 O=C(NC1C=CC=NC=1)NC1C=CC=C(C#N)C=1 DAR-DIA-23a Z195739680 Z195739680 False False
25 O=C(CC1=CNC2=NC=CC=C12)NC1C=CC=C(Cl)C=1 DAR-DIA-23a Z1400781098 False False False
26 O=C(NC1C=C(Cl)C=CC=1)NC1C=NC=CC=1 DAR-DIA-23a Z44592325 False False False
27 O=C(CC1C2C=CC=NC=2NC=1)NCC1C=C(Cl)C=CC=1 TRY-UNI-1fd Z1400783129 False False True
28 O=C(CC1C2C=CC=NC=2NC=1)NCC1C=C(C#N)C=CC=1 TRY-UNI-1fd Z1400838108 False False True
29 O=C(CC1C2C=CC=NC=2NC=1)NCC1C=CC(Cl)=CC=1 TRY-UNI-1fd Z1400780364 False False True
30 O=C(CC1C2C=CC=NC=2NC=1)NCC1C=CC(C#N)=CC=1 TRY-UNI-1fd Z1400838206 False False True
31 O=C(CC1C2C=CC=NC=2NC=1)NCC1C=C(Cl)C=CN=1 TRY-UNI-1fd Z1575152133 False False True
32 O=C(CC1C2C=CC=NC=2NC=1)NCC1C=CC(Cl)=CN=1 TRY-UNI-1fd Z1593808911 False False True
33 O=C(CC1C2C=CC=NC=2NC=1)NCC1C=CC(C#N)=CN=1 TRY-UNI-1fd Z1767447079 False False True
34 C1=C(OC)C(OC)=C(OC)C=C1NC(C(NC1=CC(OC)=C(OC)C(... ANT-LOU-17a Z62468012 False False False
I suggest we begin by sending to Enamine the ones that are not modeled after covalent fragments (last column=False), because there is some confusion regarding the design (specifically the need to include electrophiles, see Submission NIM-UNI-36e discussion)
In particular, I know for a fact that the purchasable molecule from PAT-UNK-b2d and KIM-UNI-2ee should not be ordered yet. I have not done an extensive review of any of the others.
I have checked that none of these were in the earlier fragment screens.