Topic automatically created for discussing the designs at:
https://covid.postera.ai/covid/submissions/DAV-ILL-89eb723b
Interesting concept - could you add in the benzopyran analogue and the ‘open chain’ m-Cl phenylacetic acid analogue’ particularly the later as it’s slightly more conformationally flexible, and if the THIQ needs to move a bit in the S1 pocket it will be more likely to make that possible.
Hi Ed, I’m not sure how to include David in the discussion and this does look like an interesting idea. I’d expect the the THIQ nitrogen to be predominately protonated and this is likely to compromise affinity even if the histidine side chain flips. In the cystal structures that I’ve seen the histidine [nX2] (strong HB acceptor) accepts an HB from a tyrosine OH (strong HB donor). Flipping the histidine would have the weaker HB donor (histidine [nH] donating the HB to the weaker HB acceptor (tyrosine OH).
I believe that it would be better to replace the THIQ nitrogen with oxygen (I recall that some kinase hinge binders use ether oxygen as an alternative to the more commonly used [nX2]) and I’m presuming that’s the benzopyran analog that you were referring to. For testing new ideas, I would recommend looking at the m-Chlorobenzyl analogs as you’ve suggested.
Being able to use an aliphatic P1 substituent would have a couple of advantages. First, the ring plane of the P1 substituent would tend naturally to be orthogonal with respect to the amide plane (as required for binding). Second, it would allow us to more easily exploit the amide NH as a vector for synthetic elaboration (when P1 is aromatic linking sp3 C to the amide N inverts the cis/trans geometric preference of the amide). Potential downsides are poorer fit into the S1 subsite and an additional chiral center.
Thanks for the feedback, Ed. I think the benzopyran analogue will have oxygen in the wrong position to be a hydrogen bond acceptor. I’m not sure exactly what you mean by the open chain analogue, but the more flexible group will probably lose more entropy. Still, they could be worth trying.
I’ve created another submission replacing the nitrogen with oxygen. If both are made and tested it would help us probe the importance of the histidine rotamer.
Aaagh the lack of structures is holding us back:
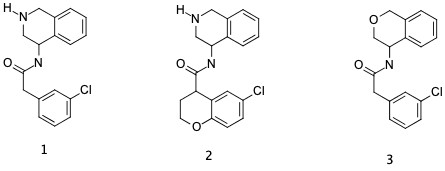
I was thinking of (1) for the “open chain” (2) for “the benzopyran”, I think Pete is suggesting (3) which given the morpholines which are used in kinases as a hingle binder instead on qunzololines etc is an interesting thought. @pwkenny @daveminh would one of you stick those ideas in? Thanks, Ed
Hi Ed, I’ll pick up 3 (and potentially some analogs) along with some other other stuff over the next couple of days. Better to generate binding modes for the submission. I’ll mention you when I submit or let you know if I conclude that it’s not worth submitting.
I’ve asked a student to do some docking of 1 & 2 and try to see which make the most sense from the standpoint of sterics and to play around with some variations to include with a submission.