https://covid.postera.ai/covid/submissions/3c79be55-6044-4966-b3bd-3eca32e15db9
The five compounds seem to all be easily synthesizable from Enamine building blocks (as Anthony nicely designed them that way).
Compounds 2, 3, and 5 look to be one step syntheses from Enamine in stock compounds. 1 and 4 look to be very doable two step syntheses. Will double check with Enamine in the morning.
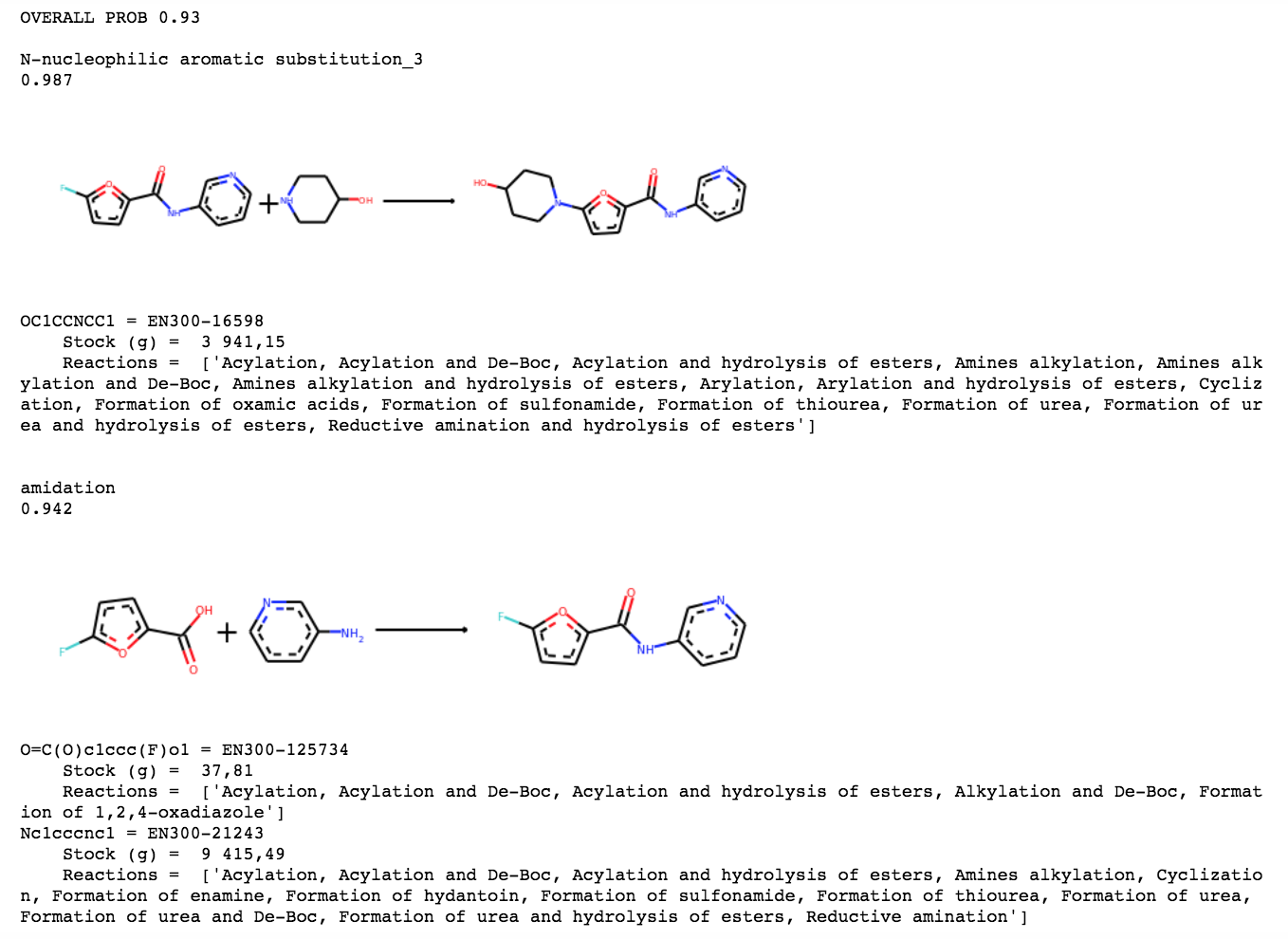
For ANT-DIA-3c7-1, Our technology finds the following route from Enamine’s building blocks: It should be an easy one step reaction ready to send off.
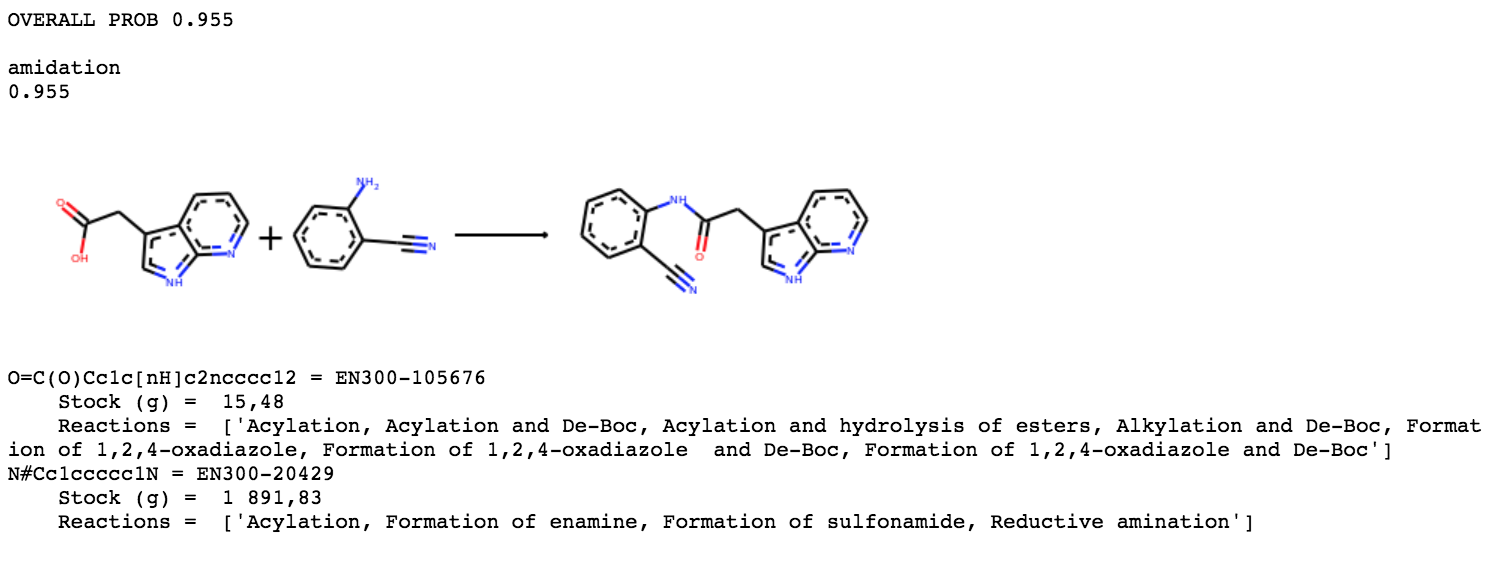
For ANT-DIA-3c7-2,
We again find a simple one step amidation from Enamine building blocks.
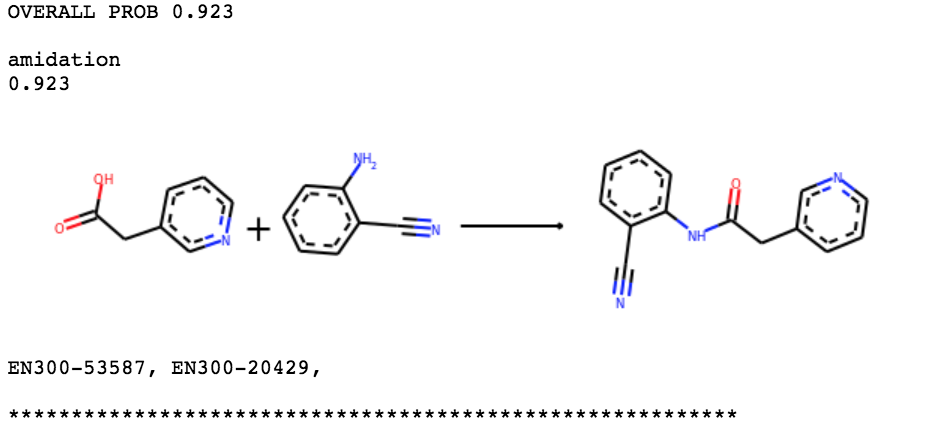
ANT-DIA-3c7-3 is a bit harder. Our algorithm finds two routes, one involving two steps with a SNAr and the other using two steps with a Buchwald-Hartwig. Unfortunately, neither is amenable to highly parallel chemistry.
This one may need a slight redesign – can work on tomorrow.
ANT-DIA-3c7-4 should hopefully be simple. 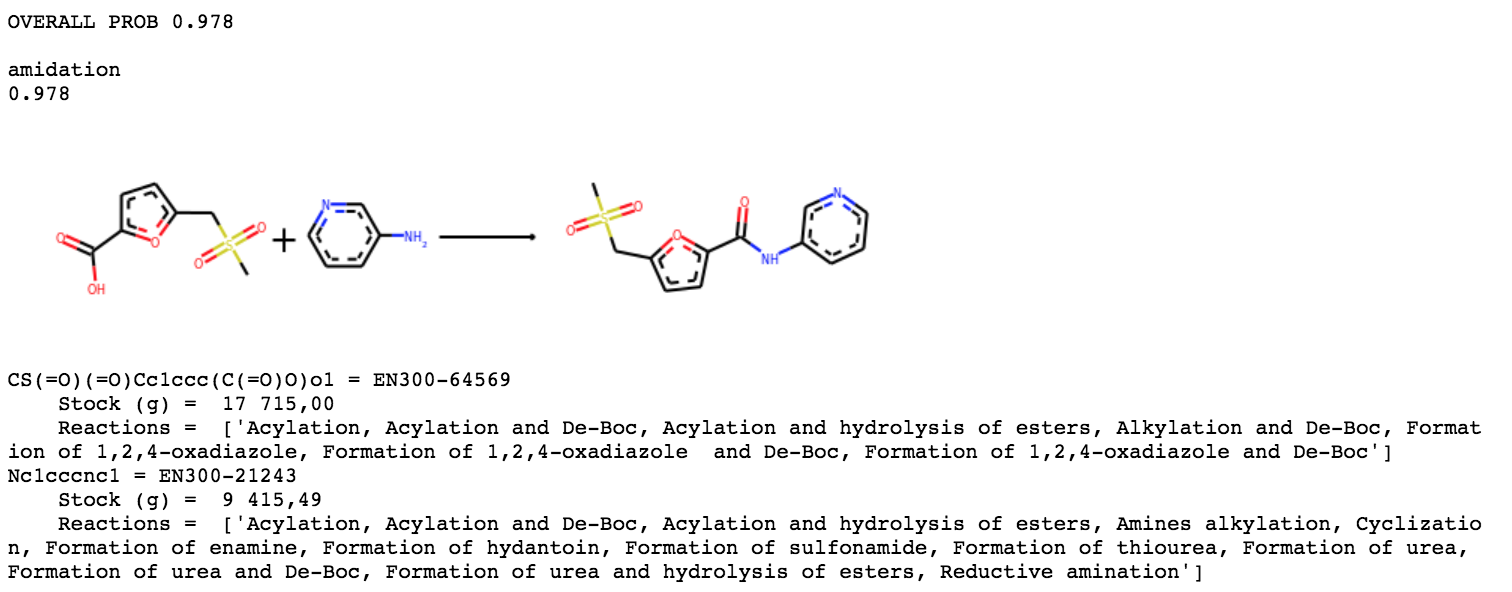
And ANT-DIA-3c7-5 should be do-able, though probably less easy than 1,2,4. One can also start with the Chlorinated, or Brominated species, but the SNAr I think works best with the fluorine species.
Sorry the routes are so ugly, I was running locally to integrate Enamine’s data quickly