bart.lenselink
Hi Willem, as we discussed I also submitted the R groups but than on the JAG-UCB scaffold.
https://covid.postera.ai/covid/submissions/ace1b61b-cdd4-44c3-a76b-110770fd18fc
Hi Willem, as we discussed I also submitted the R groups but than on the JAG-UCB scaffold.
https://covid.postera.ai/covid/submissions/ace1b61b-cdd4-44c3-a76b-110770fd18fc
Are there any plans to follow this up, it should be fruitful to explore the p5 pocket a bit more given the WaterMap + results of beta-lactam (TRY-UNI-2eddb1ff-7)?
1 replyHi @bart.lenselink, thanks for reminding me of these. I’ll look into building blocks tomorrow, and see if we can get a couple made. Definitely interest on our side in exploring that pocket more
1 replyPerfect, in that case in the mean time Ill run a reaction based enumeration on that position.
1 replyHi @bart.lenselink, I have already done that actually for Enamine building blocks with nucleophilic dispacement, Buchwald Hartwig, and Suzuki.
I’ll send them your way to save you some work!
1 replyThanks! Ill submit them for docking first.
How about the following, reaction -where the beta-lactam can be varied by other halides?:
So here’s what we did:
This is one step from Enamine
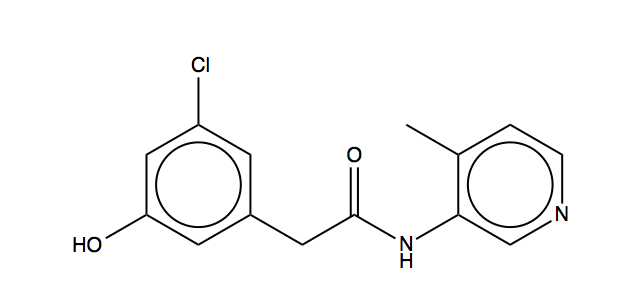
So can either do direct nucleophilic displacement of halide (we looked for SMARTS of form [CX4H2][Br,I,$(OC(=O)C),$([O][S](=[O])(=[O])[C]([F])([F])[F]),$([O][S](=[O])(=[O])c1ccc(C)cc1)] in Enamine
Or for Suzuki and Buchwald-Hartwig, we first react OH -> OTf, and then form the C-N or C-C bond. In that case, we looked for boronic esters and primary amines available at Enamine.
Hopefully that captures pretty much everything you were imagining by varying the halide?